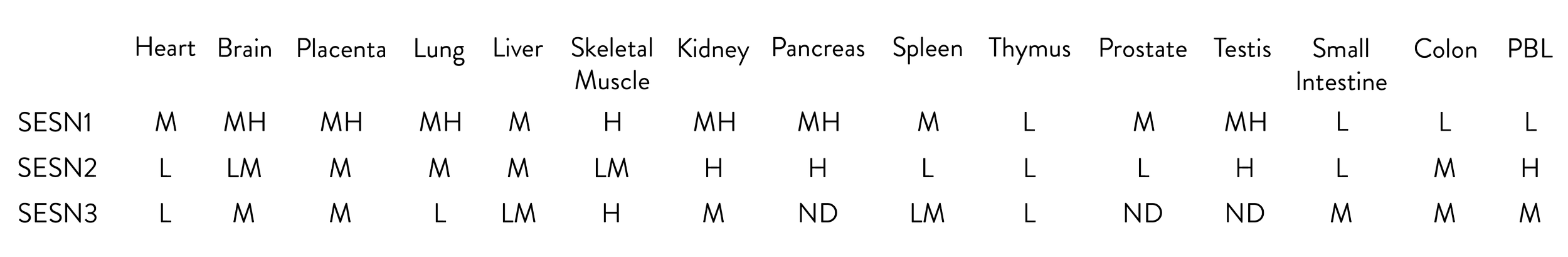What is Sestrin?
A family of animal kingdom proteins
Highly conserved – functional motifs almost identical from C. Elegans to human
Ubiquitously expressed – found in most human tissues
Stress-responsive – highly upregulated in response to cellular stress
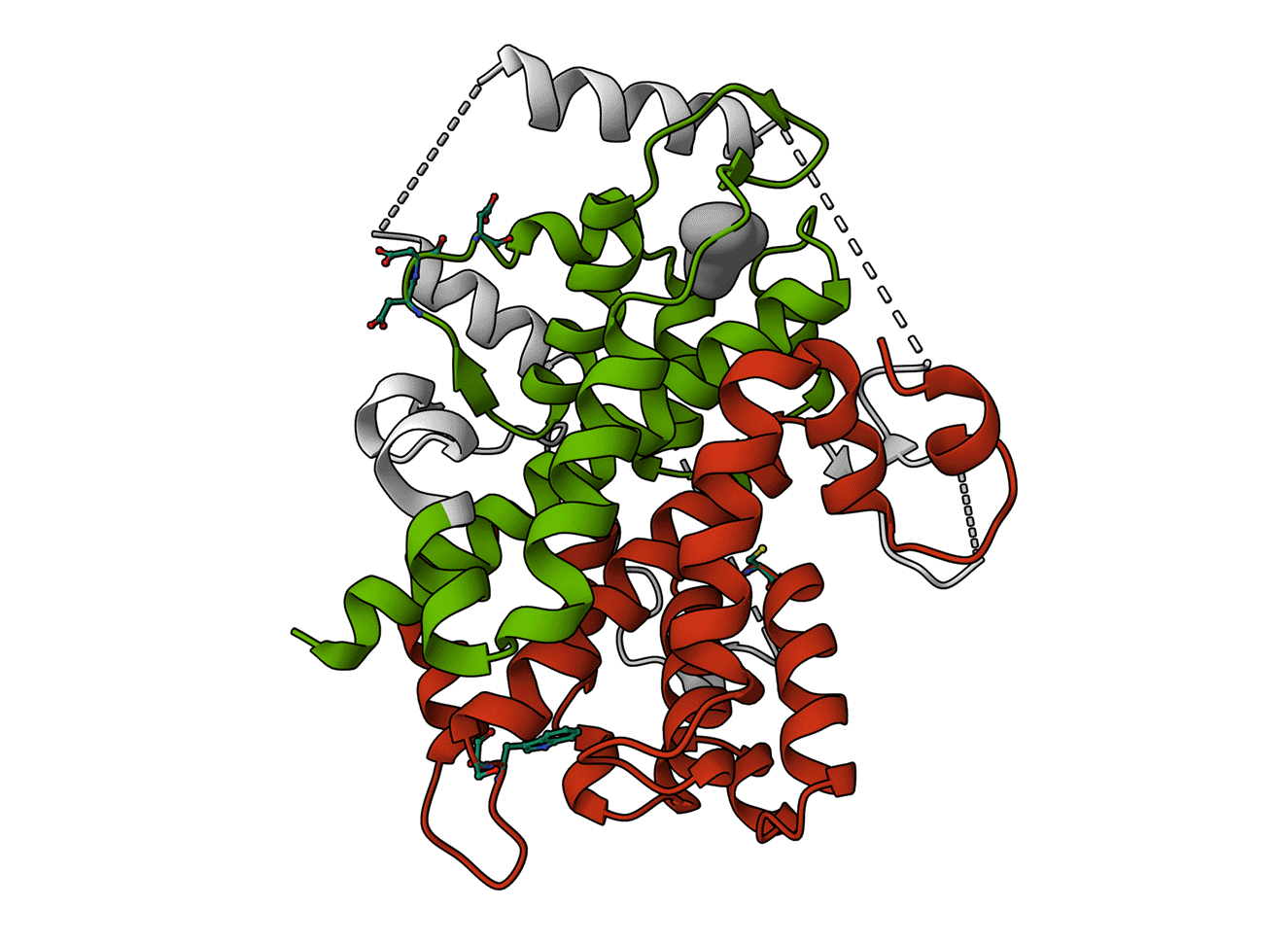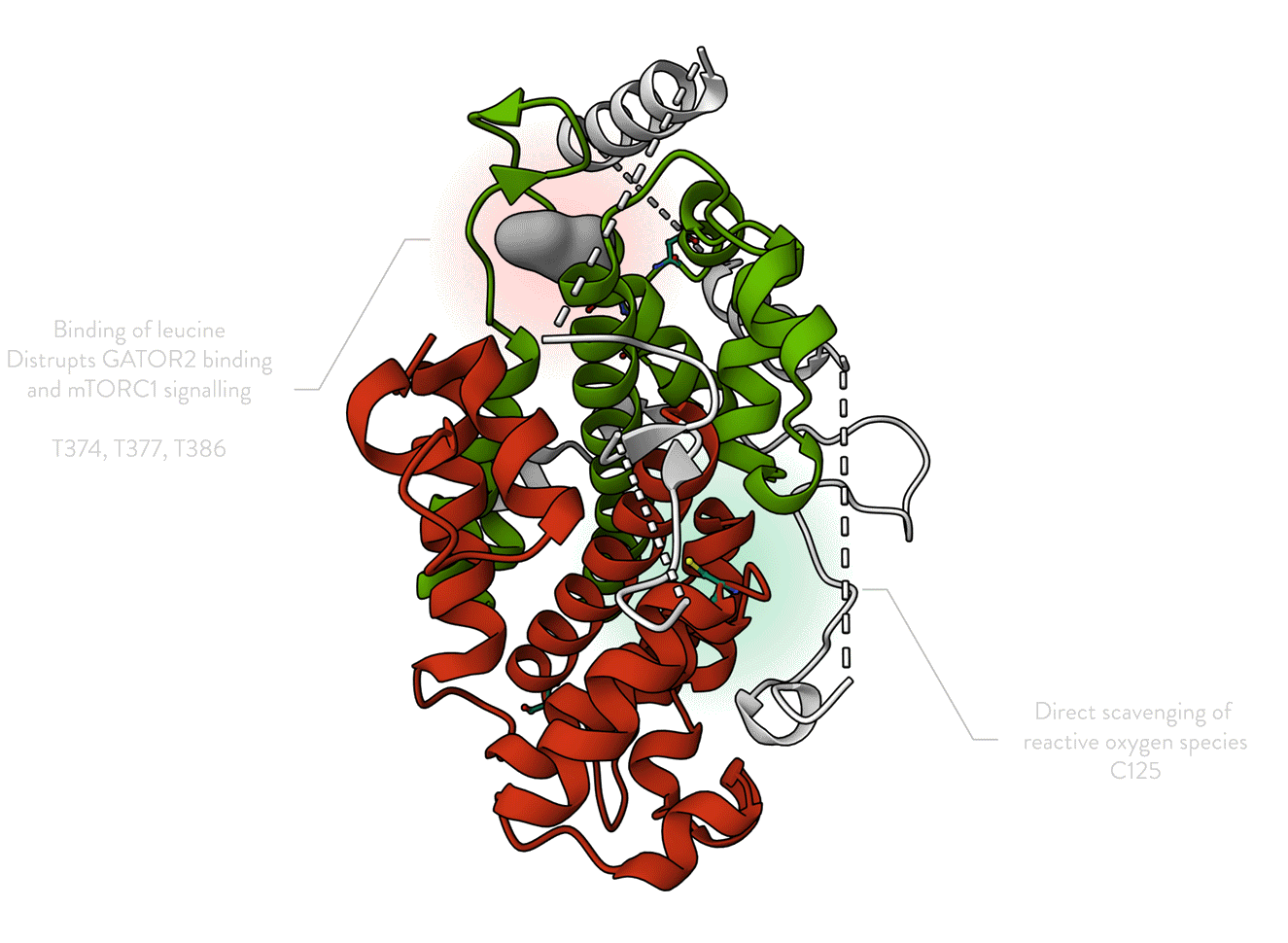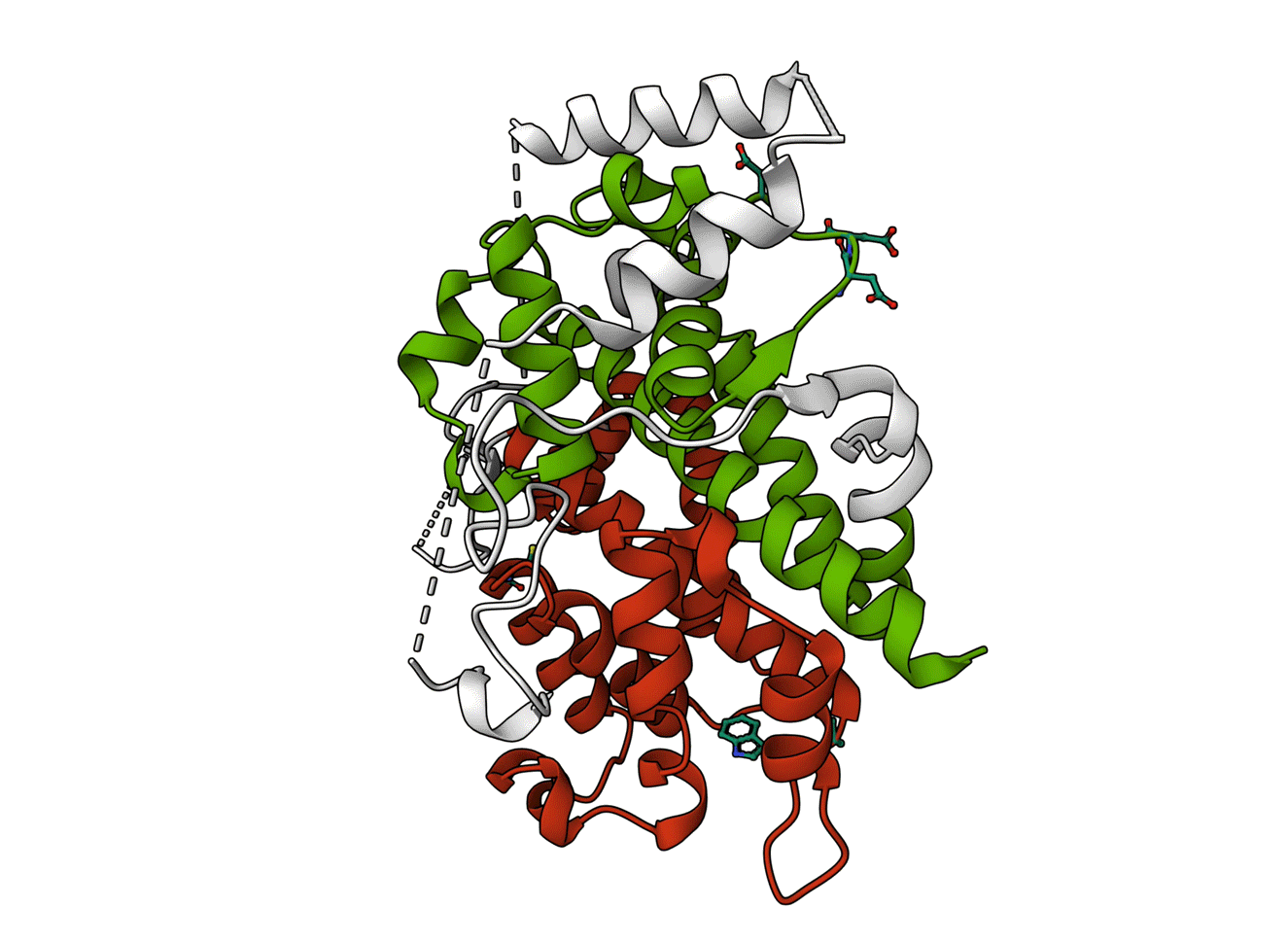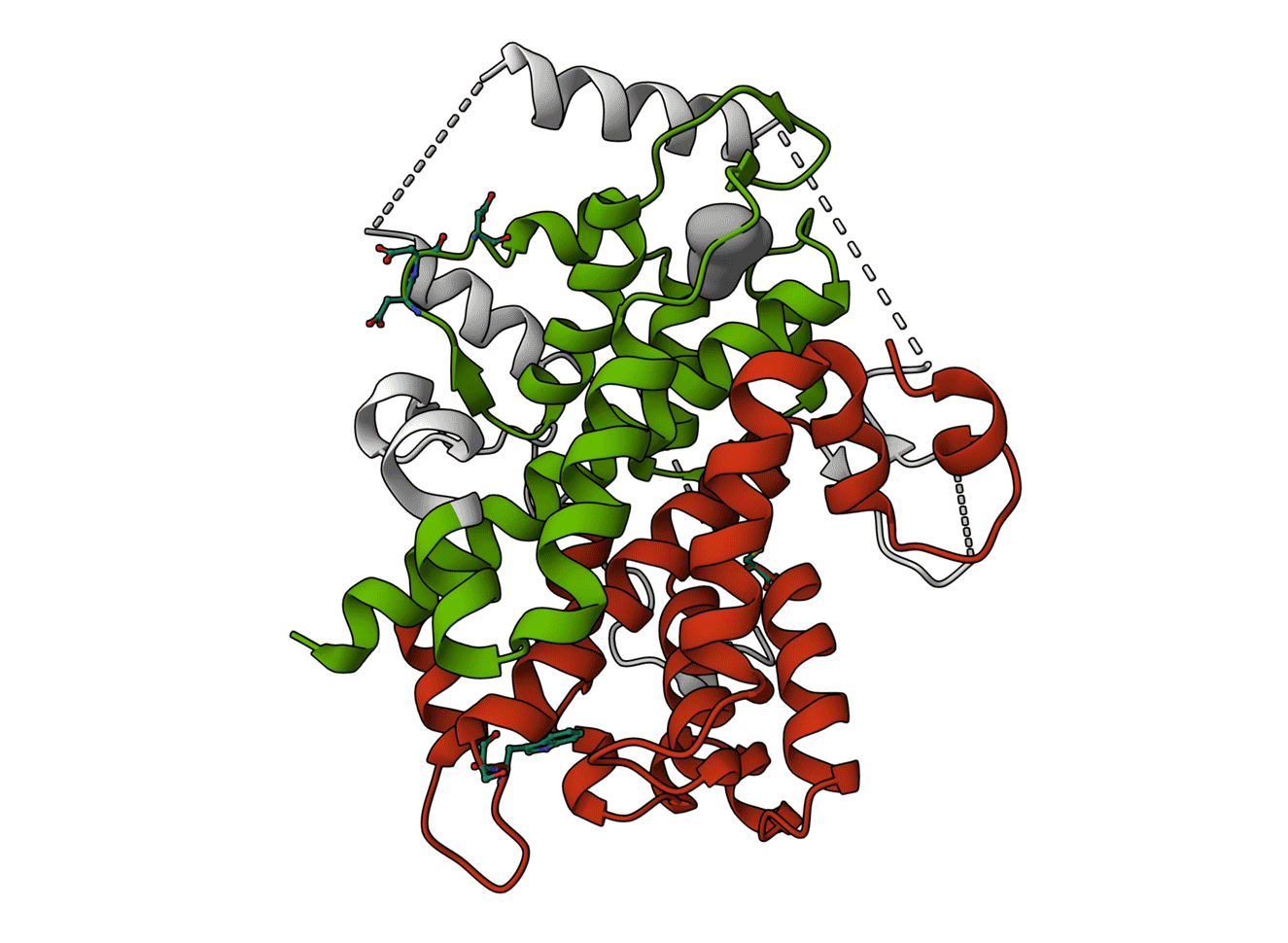
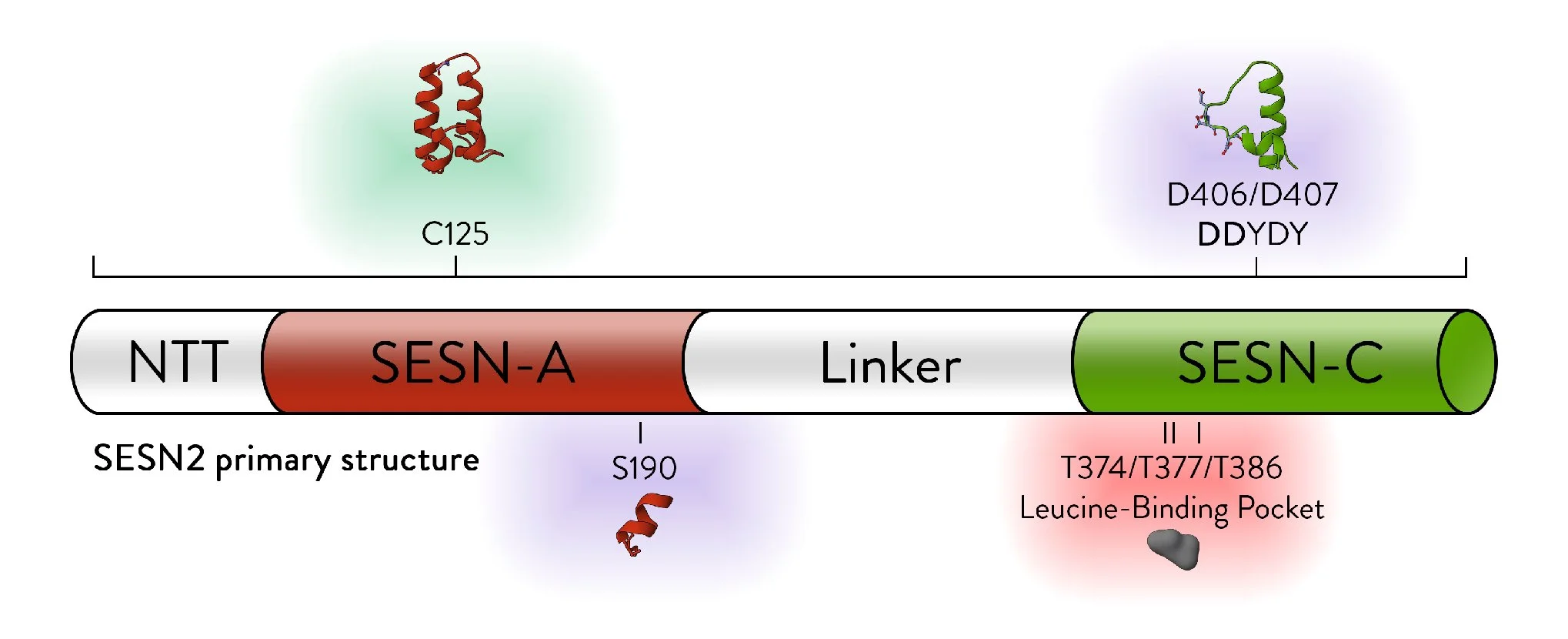
Animated Figure - The structure of SESN2
The structure is displayed from four different angles (PDB ID: 5DJ4). A ribbon cartoon of the primary structure is displayed below it. NTT—N-terminal-tail. The red, white, and green colours correspond to the domains they represent on the structure. Red—SESN-A; Green—SESN-C; White—linker. Important sites are annotated.
Key structural features:
Anti-oxidative motif (C125) that directly neutralises hydrophobic reactive oxygen species
Binding with the GATOR2 complex via multiple residues (DD406/407) – results in inhibition of mTORC1
Binding of a leucine molecule, which prevents GATOR2 binding – leucine binding pocket
Sestrin’s key biological roles:
Stops cellular protein synthesis by inhibiting mTORC1
Controls protein synthesis based on leucine levels in the cell
Eliminates reactive oxygen species
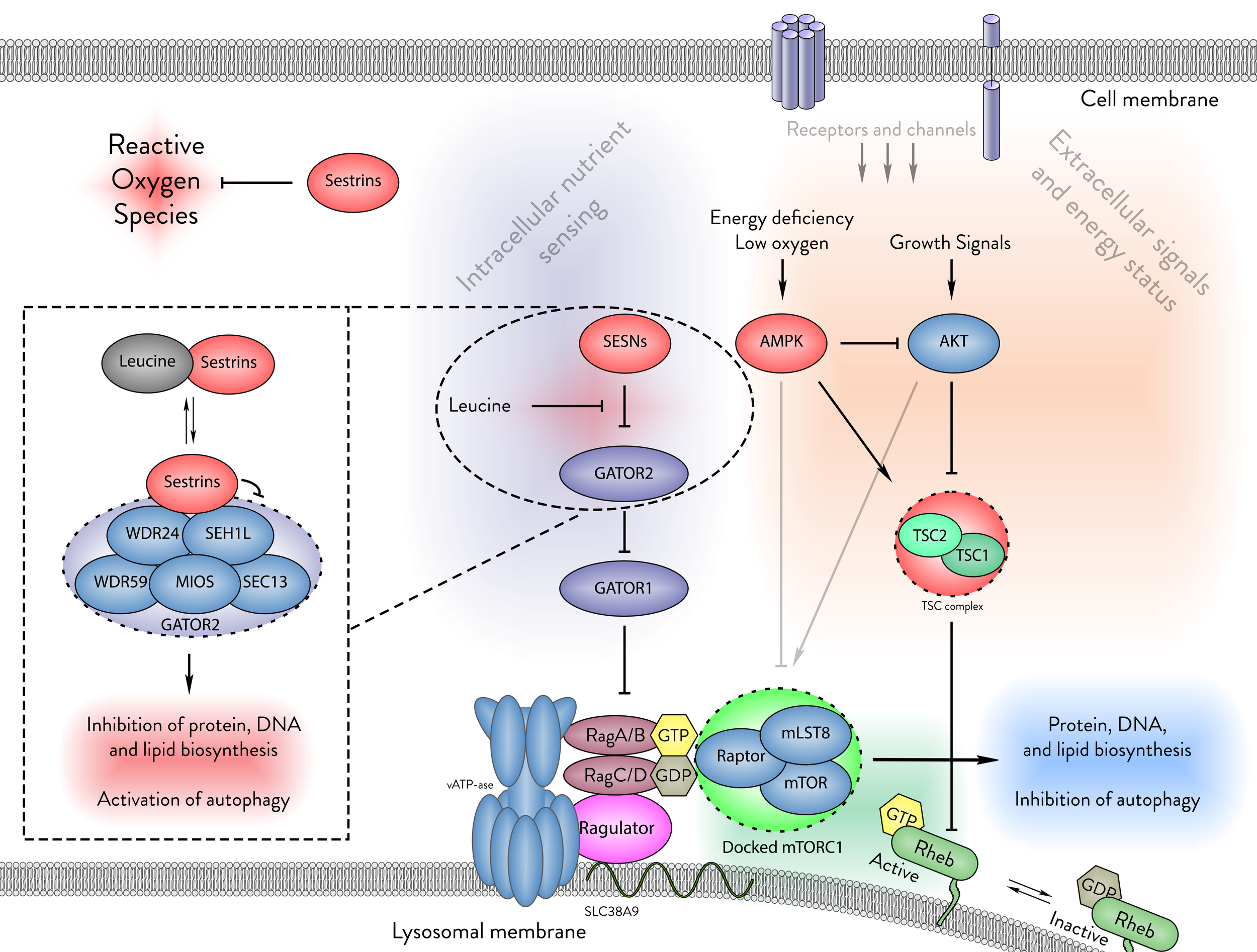
Figure - The cellular functions of Sestrins.
Sestrins play a critical role in cellular homeostasis by neutralising reactive oxygen species and sensing intracellular leucine levels. In the absence of leucine, Sestrins bind to the GATOR2 complex, thus inhibiting mTORC1. This binding prevents mTORC1 from docking on the lysosomal membrane, thereby suppressing protein, DNA, and lipid biosynthesis while promoting autophagy.
Major milestones in Sestrin research:
Discovery of SESN1 (1994) [1], SESN2 (2002) [2], SESN3 (2003) [3].
2004 first functional breakthrough - Sestrin announced to have a significant antioxidative role in the cell [4].
2008 second major breakthrough - Sestrin is an inhibitor of mTORC1 [5].
2014 mechanistic milestone - Sestrins signal to mTORC1 through the GATOR [6, 7] complexes.
2016 structural breakthrough - Crystal structure of SESN2 identifies it as a leucine sensor [8, 9].
2025 The binding of SESN2 to GATOR2 has been demonstrated via a resolved cryo-EM complex [10].
Key Sestrin upregulation signals:
DNA damage p53 [1, 2]
Oxidative stress Nrf2 [3, 4], FOXO1&3 [5, 6]
ER Stress Nrf2 [7], ATF4 [8], ATF6 [9], XBP1 [10]
Hypoxia possibly via HIF1α [11, 12]
Glutamine deprivation C/EBP [13]
Growth factor deprivation c-Jun [14]
Figure - Upregulation of Sestrins
The Sestrin family responds to a variety of cellular stresses. DNA damage upregulates SESN1&2. Oxidative damage upregulates SESN3 but can also cause upregulation of SESN1&2 via p53. ER and energy stresses can upregulate SESN2 via Nrf2 or ATF4. Hypoxia upregulates SESN2, but it is unclear whether that occurs in a HIF-1α-dependent manner.
Evolution:
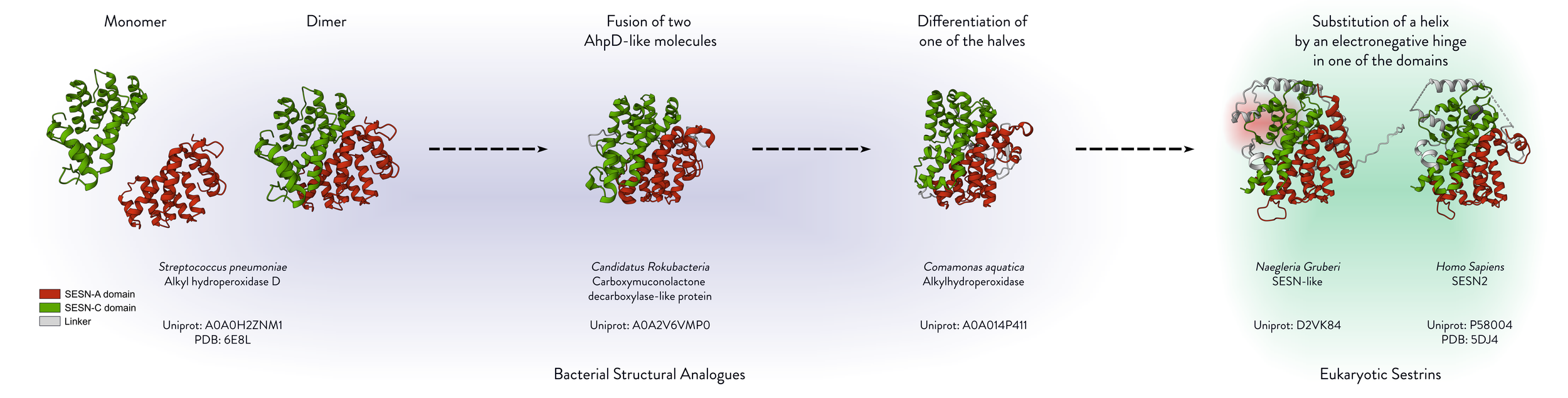
Sestrin is hypothesised to be derived from the bacterial antioxidant protein AhpD.
Its structure closely resembles a fusion of two AhpD-like structures.
One half of the protein appears to have retained its ancestral antioxidant function, while the other evolved to interact with GATOR2 and Leucine.
Figure - The two sides of Sestrins
The key structural difference between the SESN-A and SESN-C domains. This figure highlights the key structural differences between the two core domains of SESN2 (PDB ID: 5DJ4). Key differences are annotated.
Figure - The ancestry of Sestrins
The figure displays the bacterial structural analogues of Sestrins. The hypothetical evolutionary route is illustrated from left to right, beginning with the monomeric spAhpD and ending with the human SESN2. The structures of the bacterial proteins were aligned to SESN2 using PyMOL, and Mol* Viewer was used to generate cartoon representations at the same angle for visual clarity. PDB IDs are annotated, and AlphaFold-predicted structures were used where experimental structures were unavailable.
Sestrin expression:
Most tissues have a basal level of Sestrin expression.
This is hypothesised to reflect different needs for leucine sensing – different basal levels of Sestrin produce differing responses to cellular leucine changes.
Under stress conditions, Sestrin expression increases well above basal levels, triggering a strong anti-stress response by pausing protein synthesis and mitigating ROS.
Table - Relative expression of Sestrins in various tissues
The data used for this table were gathered from papers [2, 3, 11]. The relative signals for each are presented as high (H), intermediate medium (MH), medium (M), intermediate low (LM), low (L), and no data (ND). PBL – peripheral blood lymphocytes.
Graph - Modulation of mTORC1 activity by Sestrin expression in varying leucine concentrations
This graph illustrates a conceptual model where mTORC1 activity is depicted as a function of leucine concentration under varying Sestrin expression conditions. The depicted curves are arbitrary and are intended to conceptually demonstrate the hypothesised modulation of mTORC1 response by Sestrins in the presence of different leucine levels.
Illustration - Effects of Sestrin expression
1. Buckbinder L, Talbott R, Seizinger BR, Kley N: Gene regulation by temperature-sensitive p53 mutants: identification of p53 response genes. Proceedings of the National Academy of Sciences 1994, 91:10640-10644.
2. Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, et al: Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 2002, 21:6017-6031.
3. Peeters H, Debeer P, Bairoch A, Wilquet V, Huysmans C, Parthoens E, Fryns JP, Gewillig M, Nakamura Y, Niikawa N, et al: PA26 is a candidate gene for heterotaxia in humans: identification of a novel PA26-related gene family in human and mouse. Human Genetics 2003, 112:573-580.
4. Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM: Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 2004, 304:596-600.
5. Budanov AV, Karin M: p53 Target Genes Sestrin1 and Sestrin2 Connect Genotoxic Stress and mTOR Signaling. Cell 2008, 134:451-460.
6. Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim Young C, Akopiants K, Guan K-L, Karin M, Budanov Andrei V: Sestrins Inhibit mTORC1 Kinase Activation through the GATOR Complex. Cell Reports 2014, 9:1281-1291.
7. Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM: The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep 2014, 9:1-8.
8. Saxton RA, Knockenhauer KE, Schwartz TU, Sabatini DM: The apo-structure of the leucine sensor Sestrin2 is still elusive. Science Signaling 2016, 9:ra92-ra92.
9. Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM: Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351:43-48.
10. Valenstein ML, Wranik M, Lalgudi PV, Linde-Garelli KY, Choi Y, Chivukula RR, Sabatini DM, Rogala KB: Structural basis for the dynamic regulation of mTORC1 by amino acids. Nature 2025, 646:493-500.
11. Velasco-Miguel S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, Seizinger B, Kley N: PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene 1999, 18:127-137.
12. Shin BY, Jin SH, Cho IJ, Ki SH: Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radical Biology and Medicine 2012, 53:834-841.
13. Ding B, Parmigiani A, Divakaruni AS, Archer K, Murphy AN, Budanov AV: Sestrin2 is induced by glucose starvation via the unfolded protein response and protects cells from non-canonical necroptotic cell death. Scientific Reports 2016, 6:22538.
14. Chen C-C, Jeon S-M, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N: FoxOs Inhibit mTORC1 and Activate Akt by Inducing the Expression of Sestrin3 and Rictor. Developmental Cell 2010, 18:592-604.
15. Hagenbuchner J, Kuznetsov A, Hermann M, Hausott B, Obexer P, Ausserlechner MJ: FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. Journal of Cell Science 2012, 125:1191-1203.
16. Hetz C: The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature Reviews Molecular Cell Biology 2012, 13:89-102.
17. Garaeva AA, Kovaleva IE, Chumakov PM, Evstafieva AG: Mitochondrial dysfunction induces SESN2 gene expression through Activating Transcription Factor 4. Cell Cycle 2016, 15:64-71.
18. Jegal KH, Park SM, Cho SS, Byun SH, Ku SK, Kim SC, Ki SH, Cho IJ: Activating transcription factor 6-dependent sestrin 2 induction ameliorates ER stress-mediated liver injury. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2017, 1864:1295-1307.
19. Saveljeva S, Cleary P, Mnich K, Ayo A, Pakos-Zebrucka K, Patterson JB, Logue SE, Samali A: Endoplasmic reticulum stress-mediated induction of SESTRIN 2 potentiates cell survival. Oncotarget; Vol 7, No 11 2016.
20. Essler S, Dehne N, Brüne B: Role of sestrin2 in peroxide signaling in macrophages. FEBS Letters 2009, 583:3531-3535.
21. Olson N, Hristova M, Heintz NH, Lounsbury KM, van der Vliet A: Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction. American Journal of Physiology-Lung Cellular and Molecular Physiology 2011, 301:L993-L1002.
22. Byun J-K, Choi Y-K, Kim J-H, Jeong JY, Jeon H-J, Kim M-K, Hwang I, Lee S-Y, Lee YM, Lee I-K, Park K-G: A Positive Feedback Loop between Sestrin2 and mTORC2 Is Required for the Survival of Glutamine-Depleted Lung Cancer Cells. Cell Reports 2017, 20:586-599.
23. Zhang X-Y, Wu X-Q, Deng R, Sun T, Feng G-K, Zhu X-F: Upregulation of sestrin 2 expression via JNK pathway activation contributes to autophagy induction in cancer cells. Cellular Signalling 2013, 25:150-158.